The US Food and Drug Administration (FDA) has approved iloprost, the inaugural treatment designed specifically for severe frostbite.
This medication, now authorized for use, presents promising potential in safeguarding the extremities of adults susceptible to amputation.
Annually, the Minnesota Department of Health monitors the health implications stemming from extreme cold weather conditions. Despite the relatively milder winter experienced this year, frostbite continues to pose a significant threat, leading to numerous hospitalizations among Minnesotans.
- California RESIDENTS To RECEIVE $500 MONTHLY PAYMENTS In New Guaranteed Income Program: Here Is Who Is Eligible
- Us Job Market Booms With 272,000 New Jobs, But Unemployment Rises Slightly To 4.0%
- Credit Scores Of 720+ See Improved Loan Rates This Week
- Examining The Facts: Fact Check On IRS $8700 Stimulus Check Eligibility & Payment Dates
- Gas Prices On Downward Trend As Fourth Of July Road Trips Approach: AAA
In the exceptionally cold winter of 2022, emergency room visits and hospital admissions due to frostbite witnessed a notable surge.
Cases of superficial frostbite totaled 1,055, while severe frostbite resulting in tissue necrosis amounted to 114.
However, the subsequent year saw a substantial decline in frostbite-related hospitalizations, with superficial cases dropping to 503 and severe frostbite injuries reducing to 70.
Iloprost: Vasodilator Enhancing Blood Flow
Iloprost, the active component in Aurlumy, functions as a vasodilator, a class of medication that widens blood vessels to facilitate blood flow and prevent clot formation.
Initially sanctioned in 2004 for the management of pulmonary arterial hypertension, iloprost aids in enhancing circulation.
In a controlled clinical trial, researchers evaluated 47 adult subjects afflicted with severe frostbite, all of whom were administered intravenous aspirin in addition to standard medical care.
Participants were randomly divided into three cohorts: the first group received intravenous iloprost for six hours daily for up to six days, while the second and third groups were administered alternative medications not approved for frostbite treatment, with the second group receiving iloprost alongside these medications and the third group without iloprost.
After seven days, bone scans were conducted to assess the likelihood of requiring amputation of at least one digit.
Notably, none of the patients in the first group, which solely received iloprost, exhibited indications necessitating amputation on their bone scans. In contrast, 19% of patients in the second group and 60% in the third group displayed such indications.
Frostbite Symptoms and Progression
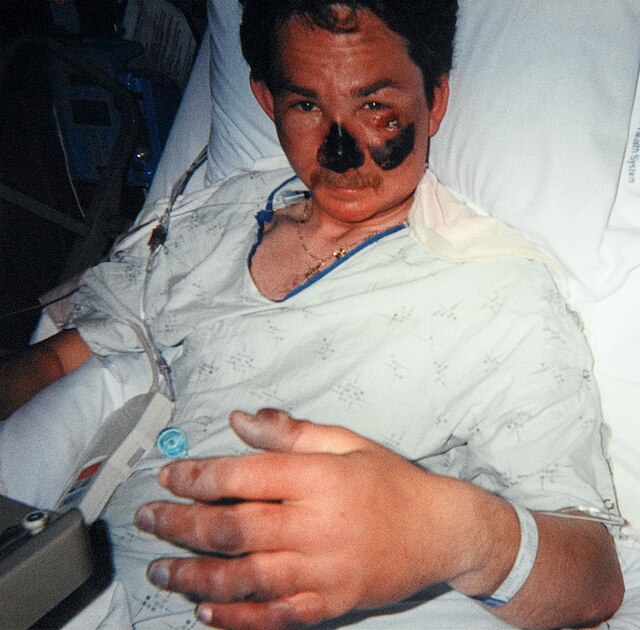
Frostbite represents an injury induced by exposure to cold temperatures, resulting in damage to both the skin and underlying tissues.
Common treatment measures involve immersing the affected skin in warm water or applying blankets to the afflicted area.
The progression of frostbite generally unfolds across three distinct stages, culminating in severe frostbite characterized by numbness and the development of hardened, blackened skin indicative of cellular necrosis.
According to the Cleveland Clinic, notable symptoms include the formation of large blisters on the skin within a day or two following cold exposure, with the potential for permanent damage to the affected skin.
If the underlying tissues have undergone freezing and blood circulation has ceased, surgical removal of the frostbitten extremity may be necessary.
